Slide
Enteris BioPharma rebrands to MOD3 Pharma
Enteris BioPharma, a fully integrated contract development and manufacturing organization, today announced that the company is changing its name to MOD3 Pharma.
A wholly owned subsidiary of SWK Holdings Corp. (SWKH), MOD3 Pharma continues to build on its strong growth agenda by refocusing its service portfolio to supporting investigational drugs in the development of Orally Inhaled and Nasal Drug Products (OINDP).
Slide
Your Catalyst to Clinic
MOD3Pharma combines decades of CMC experience with a laser focus on Orally Inhaled and Nasal Drug Products (OINDP) to transform your project into a clinic-ready proposition.
Using a stage-appropriate methodology within the Module 3 framework, our experts work collaboratively with you to manage risk, mitigate delay and reduce cost while ensuring your OINDP product’s quality, safety, and efficacy.
Slide
Finding the Right Formulation
With experience of oral solid, nasal and Injectable dosage forms as well as many OINDPs, MOD3 Pharma brings deep insight and intelligence to formulation science.
We have the technical capabilities and cutting-edge technologies to enhance the bioavailability of even the most challenging APIs.
Slide
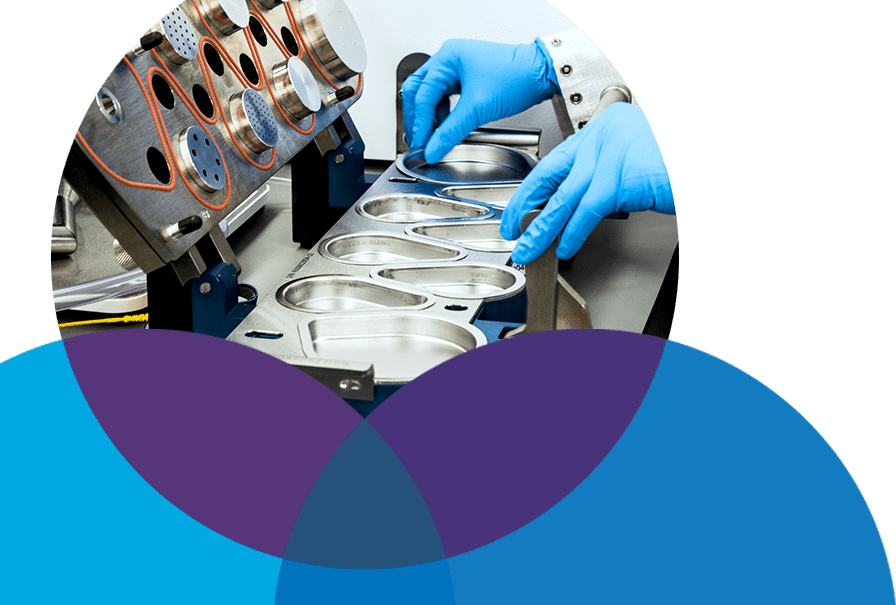
Equipped for Quality Manufacturing
Navigating the supply of your drug product for early phase clinical trials requires a team of scientists with a passion for drug development and the creativity to solve problems decisively. You need a team behind you that have seen it and done it before. Many times before.
At MOD3 Pharma, our seasoned experts start brilliantly with the end in mind, using a stage-appropriate framework to answer every complex element within Module 3. Together, we will transform your project into a clinic-ready proposition.




Slide
Enteris BioPharma rebrands to MOD3 Pharma
Enteris BioPharma, a fully integrated contract development and manufacturing organization, today announced that the company is changing its name to MOD3 Pharma.
A wholly owned subsidiary of SWK Holdings Corp. (SWKH), MOD3 Pharma continues to build on its strong growth agenda by refocusing its service portfolio to supporting investigational drugs in the development of Orally Inhaled and Nasal Drug Products (OINDP).
Slide
Your Catalyst to Clinic
MOD3Pharma combines decades of CMC experience with a laser focus on Orally Inhaled and Nasal Drug Products (OINDP) to transform your project into a clinic-ready proposition.
Using a stage-appropriate methodology within the Module 3 framework, our experts work collaboratively with you to manage risk, mitigate delay and reduce cost while ensuring your OINDP product’s quality, safety, and efficacy.
Slide
Finding the Right Formulation
With experience of oral solid, nasal and Injectable dosage forms as well as many OINDPs, MOD3 Pharma brings deep insight and intelligence to formulation science.
We have the technical capabilities and cutting-edge technologies to enhance the bioavailability of even the most challenging APIs.
Slide
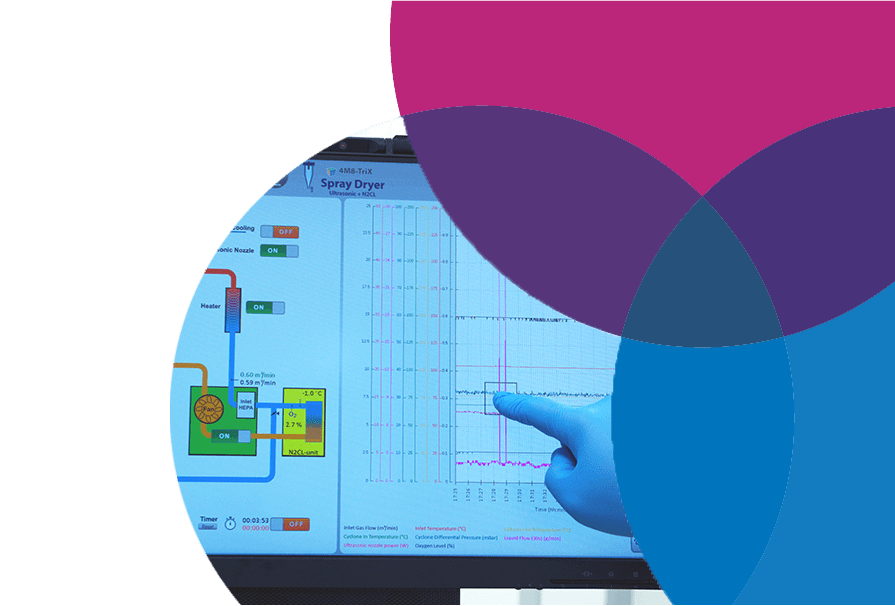
Equipped for Quality Manufacturing
With specialized equipment for manufacturing liquid and nasal OINDPs, MOD3 Pharma has the technology to turn your formulation into a drug product.
We put the right technology to work for you to speed your drug to the clinic and ensure you have the data to write Module 3 of your IND or IMPD. It's in our name!




Slide
Enteris BioPharma rebrands to MOD3 Pharma
Enteris BioPharma, a fully integrated contract development and manufacturing organization, today announced that the company is changing its name to MOD3 Pharma.
A wholly owned subsidiary of SWK Holdings Corp. (SWKH), MOD3 Pharma continues to build on its strong growth agenda by refocusing its service portfolio to supporting investigational drugs in the development of Orally Inhaled and Nasal Drug Products (OINDP).
Slide
Your Catalyst to Clinic
MOD3Pharma combines decades of CMC experience with a laser focus on Orally Inhaled and Nasal Drug Products (OINDP) to transform your project into a clinic-ready proposition.
Using a stage-appropriate methodology within the Module 3 framework, our experts work collaboratively with you to manage risk, mitigate delay and reduce cost while ensuring your OINDP product’s quality, safety, and efficacy.
Slide
Finding the Right Formulation
With experience of oral solid, nasal and Injectable dosage forms as well as many OINDPs, MOD3 Pharma brings deep insight and intelligence to formulation science.
We have the technical capabilities and cutting-edge technologies to enhance the bioavailability of even the most challenging APIs.
Slide
Equipped for Quality Manufacturing
With specialized equipment for manufacturing liquid and nasal OINDPs, MOD3 Pharma has the technology to turn your formulation into a drug product.
We put the right technology to work for you to speed your drug to the clinic and ensure you have the data to write Module 3 of your IND or IMPD. It's in our name!
Risk. Time. Cost. The trifecta of challenges that can keep you awake at night. Because your project matters – it really matters, not just to you, but to the patients that will rely on your therapy to make their lives better.
Navigating the supply of your drug product for early phase clinical trials requires a team of scientists with a passion for drug development and the creativity to solve problems decisively. You need a team behind you that have seen it and done it before. Many times before.
At MOD3 Pharma, our seasoned experts start brilliantly with the end in mind, using a stage-appropriate framework to answer every complex element within Module 3. Together, we will transform your project into a clinic-ready proposition.

SCHEDULE A MEETING
We really want to talk to you about your next OINDP product.
Let us be your catalyst to clinic.
Ask Us a Question
Our team relish finding answers to questions that haven’t been thought of yet.